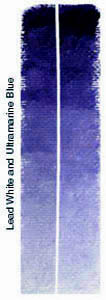
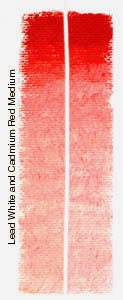
Pigment Test6 Month Exposure to South Sunlight - Summer The test consisted of two parts under two
conditions: For the following tests, oil colors were mixed and applied to acrylic primed canvas. When dry, the strips were cut in half vertically. A.) For tests 1a and 2a, one half was kept in a drawer in my studio; the other half was attached to a board and taped in a south facing window where it was exposed to the summer sun from April 15th, 2000 until October 14th, 2000. The experiment took place in Fort Wayne, Indiana, USA. The northern latitude should be taken into consideration. The results may differ when exposed to the stronger rays of the sun near the equator. B.) For test 1b and 2b, the strips that had been exposed to the sun during the summer were set outside and exposed to the winter weather and further south sun from the end of October, 2000, until April 14th, 2001. The control strips were taken out of the dark drawer and exposed to direct sun for several weeks and then to normal studio lighting. TEST ONE AExposure of Ultramarine Blue and Lead White mix and Exposure of Cadmium colors immixed with Lead White. Over a six month period all colors remained pure with no darkening of the exposed strips (right) and no apparent chalking. Discoloration in the form of yellowing did occur on the control strips (left) which were stored in a dark drawer. This is a common occurance when paintings using a linseed oil binder are stored in the dark. The yellowed strips can be cleansed by exposure to sunlight for a brief period. Beyond this yellowing of the control strips, the colors show no sign of variation between the control strips and the exposed halves. Any chemical reaction between sulfides and lead should have occured within the test period. The oil binder does appear to prevent the darkening which occurs with an aqueous medium when combining lead and sulfur or sulfides. No chalking is yet visible with the cadmiums in linseed oil when tinted with white. Whether the chalking that occurs in commercial paints containing cadmiums is due to a different binder (such as acrylic), or the effect just takes a longer period of time to become noticable is yet to be determined. However, the use of cadmiums can be examined in museums worldwide where paintings have been hanging for 80 to 100 years. An ASTM I rating for lightfastness is for 150 years under museum conditions, which would indicate that cadmiums can be expected to last in the oil painting medium for at least this long under ideal conditions. Also, the use of "Chemically Pure Cadmium" in artists oil colors would eliminate contaminates which appear to have been the main culprit in discoloration in the 19th century. Many artists may, therefore, wish to continue to use these colors rather than switch to newer synthetics which are of more recent production and have not yet stood the test of time in practical use, and which do not have the opacity of the cadmium colors. TEST ONE BExposure to sunlight remedied the yellowing of control strips that had occurred while they were stored in the dark, returning the canvas to bright white and colors to clean hues. (not shown.) Test strips exposed to the winter weather (rain, snow, ice, wind, and subfreezing temperatures) remarkably retained their color without any noticable discoloration. Some yellowing (or shadowing) which may appear on the scans are do to curling of the canvas strips and are the product of the scanning process, rather than to the test. Such extreme conditions should have produced discoloration if such was to occur but none is apparent on the original color strips.
TEST TWOA.) The second test to examine lightfastness of synthetic dye-color replacements for the above sulfur-containing pigments acheived similar results. Colors remained pure, with no fading or discoloration over the course of the experiment, except for the slight yellowing apparent on the test strips on the left due to their being stored in a dark drawer. However, due to the higher oil content of synthetic dyes compared to pigments, they are better used for top layer painting and glazing in the indirect method, or use in alla prima painting where layering of the paint is a non-issue. B.) Exposure to winter weather also produced similar results as to the sulfide mixes - there was no noticable color shifts on the test strips and yellowing of the controls was reversed with exposure to sunlight.
Info from: SANDERS-STUDIOS.COM
|